Biological Science 2015/11/13
Highlights
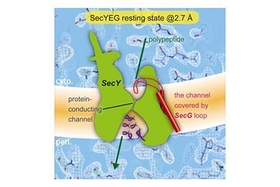
・Crystal structures of full-length and peptide-bound SecYEG were determined
・The cytoplasmic loop of SecG covers the protein-conducting channel
・The cytoplasmic loop of SecG blocks protein translocation
Summary
The bacterial SecYEG translocon functions as a conserved protein-conducting channel. Conformational transitions of SecYEG allow protein translocation across the membrane without perturbation of membrane permeability. Here, we report the crystal structures of intact SecYEG at 2.7-A resolution and of peptide-bound SecYEG at 3.6-A resolution. The higher-resolution structure revealed that the cytoplasmic loop of SecG covers the hourglass-shaped channel, which was confirmed to also occur in the membrane by disulfide bond formation analysis and molecular dynamics simulation. The cytoplasmic loop may be involved in protein translocation. In addition, the previously unknown peptide-bound crystal structure of SecYEG implies that interactions between the cytoplasmic side of SecY and signal peptides are related to lateral gate opening at the first step of protein translocation. These SecYEG structures therefore provide a number of structural insights into the Sec machinery for further study.
For more information,
http://bsw3.naist.jp/eng/researchtopics/2015/20151113.html