Materials Science 2012/06/27
Professor Michio Fujiki, Laboratory of Advanced Polymer Science, Graduate School of Materials Science
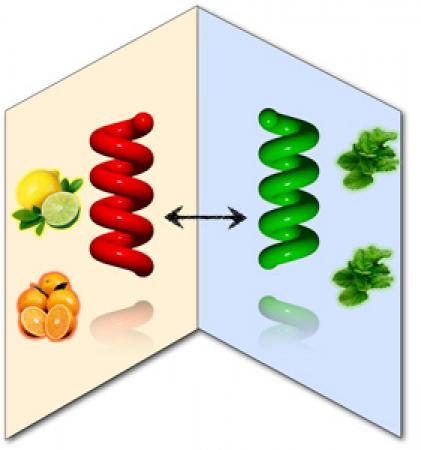
Limonene, producing in orange peel and mint leaf, is well-known as one of chiral bioresources.
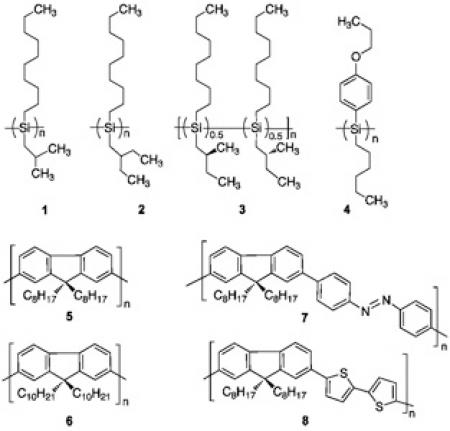
Recently, we demonstrated that three kinds of optically active Si-Si bonded polymers (polysilanes) (1-3) can be
generated from the corresponding optically inactive polysilanes with the help of limonene and methanol
cosolvent [1]. The cosolvent acts as a cocktail of mirror-symmetry-breaking solvent, leading to optically active
polymers with handedness without chiral catalysts and/or chiral substituents within 10 sec at room temperature.
The concept of the solvent chirality transfer with chiral alcohols, though very expensive, was originally proven
by the use of a very specific polysilane (4) a decade ago [2].
Recently, we found that, in place of the chiral alcohols, inexpensive limonene can generate very efficiently several
optically active pi-conjugated polymers from the corresponding optically inactive polymers (5-8) [3-5].
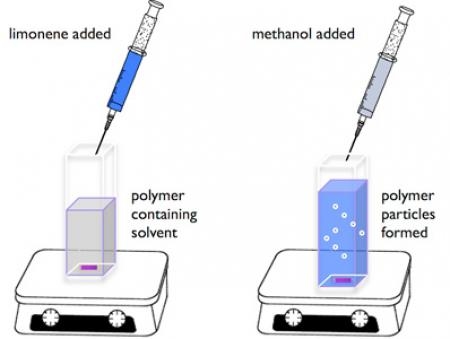
More recently, we demonstrated the scope-and-limitation of the limonene chirality transfer to generate optically active
polymer particles from the corresponding optically inactive starting materials [6].
A series of our outcomes [1-6] might open the door to innovative, environmentally friendly, inexpensive solution
process of various optically active functional polymers even from commodity polymers in near future.
Refs.
[1] Y. Nakano, F. Ichiyanagi, M. Naito, Y. Yang, M. Fujiki, Chem. Commun., 48, 6636-6638 (2012).
[2] H. Nakashima, J. R. Koe, K. Torimitsu, M. Fujiki, J. Am. Chem. Soc., 123, 4847-4848 (2001).
[3] Y. Kawagoe, M. Fujiki, Y. Nakano, New J. Chem., 34, 637-647 (2010).
[4] Y. Nakano, Y. Liu, M. Fujiki, Polym. Chem., 1, 460-469 (2010).
[5] W. Zhang, K. Yoshida, M. Fujiki, X. Zhu, Macromolecules, 44, 5105-5111 (2011).
[6] M. Fujiki, A. J. Jalilah, N. Suzuki, M. Taguchi, W. Zhang, M. M. Abdellatif, N. Nomura,
RSC Adv.DOI:10.1039/C2RA20430D (2012).