Materials Science 2015/01/08
Historically, an idea of absolute asymmetric synthesis (AAS), meaning synthesis of desired chiral molecule (one of two mirror image molecules) using circularly polarized light (CPL) source without any chemical influence, was first inferred by French scientist, Le Bel in 1874 and Dutch scientist, van't Hoff in 1894, independently. This conjecture prompted German chemists, W. Kuhn and E. Brown to challenge the first successful experimental demonstration of AAS, though ultra tiny yield in 1929. These pioneering theoretical and experimental works led to many researchers over 150 years who are interested in AAS because of no need of any specific, expensive chemicals. However, researchers had long a concrete belief that left-handed CPL source produces left-handed molecule preferentially or vice versa, because handedness of the resulting chiral molecule is determined by hand of CPL source, but independent of refractive index of solvent used so far. In addition, the typical yield of chiral product was only 0.1-1 %. This ultra-low yield is very inefficient, compared to asymmetric synthesis using chiral chemical reagents.
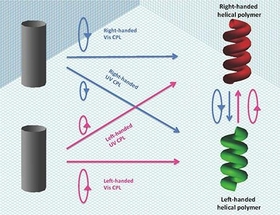
Prof. Michiya Fujiki and his coworkers found that, using chain-like green-emitting pi-conjugated polymer (PF8T2), (i) handedness of the resulting PF8T2 aggregates is determined by CPL source irradiation wavelength (UV or visible region) and its handedness. When right-CPL source in UV region is used, the resulting PF8T2 was right-handed helix, but right-CPL source in visible region is employed, the resulting PF8T2 was oppositely left-handed helix and vice versa, moreover, (ii) by precisely controlling refractive index of inexpensive common organic solvent (≈1.4, JPY 100 only per liter), the yield of PF8T2 helix increased upto 10%, that is the highest value among the heretofore reported AAS experiments.
Thus, by finely controlling four factors involving (i) wavelength of CPL source, (ii) hand (left or right) of CPL source, (iii) irradiation time of CPL source and (iv) refractive index of solvents , we can program to generate right- and left-handed helical polymers, followed by re-program (re-set) and invert helix sense. These results should lead to various photoluminescent polymer materials and substances, leading to re-writable optical memory, efficient erase, long-term storage, and efficient switching properties with massless CPL source only. Furthermore, the origin of handedness of L-amino acids and D-sugars may be interpreted to wavelength and handedness of CPL source existing in our Universe. For example, when left (or right)-handed CPL source from Universe was irradiated to Earth, shorter wavelength in UV region generates D-sugars preferentially, while longer wavelength in UV region generates L-amino acids preferentially.
In the future, we will try to generate helical polymers and chiral molecules in 100 % yield on Earth driven by CPL, anti-neutrinos, spinning electron beams, and magnetic and electric fields. This might be the ultimate goal in the community of chirality-related chemistry and physics over the last one-and-half centuries.