Materials Science 2017/09/01
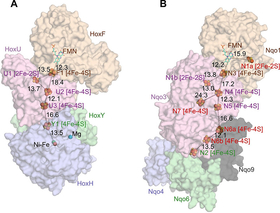
NAD+-reducing soluble [NiFe]-hydrogenase is phylogenetically related to NADH:quinone oxidoreductase (complex I) in the respiratory chain, but the geometrical arrangements of the subunits and FeS clusters of NAD+-reducing soluble [NiFe]-hydrogenase have been unclear. In this work, the crystal structures of NAD+-reducing soluble [NiFe]-hydrogenase in the oxidized and reduced states are elucidated, together with spectroscopic data. The cluster arrangement was found to be similar to that of complex I, but the subunits orientation was different. This feature supports the hypothesis that subunits evolved as prebuilt modules. The oxidized active site was found to include a six-coordinate Ni, which is unprecedented for hydrogenases. The unusual coordination geometry of the air-oxidized active site would prevent O2 from accessing the site and so may protect against irreversible oxidation. These results provide the mechanism for how a hydrogenase protects its active site, and the information may be useful for future use of hydrogenase, as well as its model compounds, for H2 production.
Paper title and author(s)
- Title: "Structural basis of the redox switches in the NAD+-reducing soluble [NiFe]-hydrogenase".
- Author(s): Y. Shomura, M. Taketa, H. Nakashima, H. Tai*, H. Nakagawa, Y. Ikeda, M. Ishii, Y. Igarashi, H. Nishihara, K-S. Yoon, S. Ogo, S. Hirota*, Y. Higuchi
* NAIST - Publication: Science (online)
- DOI: 10.1126/science.aan4497